Measuring the Zeta Potential of Highly Concentrated Fat Emulsion
2022-01-10Application Note
BeNano 180 Zeta accurately measures concentrated lipid emulsions. Dilute or titrate for true zeta potential; high conc. may mislead.
| Product | BeNano Series |
| Industry | Pharmaceuticals |
| Sample | Fat Emulsion |
| Measurement Type | Zeta Potential |
| Measurement Technology |
Jump to a section:
Introduction
What is zeta potential? When particles are suspended in a liquid medium, the surface charges attract the counterions in the liquid to attach to the particle surface, forming a firmly attached inner Stern layer, and an extended outer layer with a boundary of slipping plane. The ions and solvent in the shear layer move in the medium as an integrated whole when subjected to external force (i.e., electric field force). The potential at the slipping plane is defined as the zeta potential.
The zeta potential of particles in aqueous suspension depends on the chemical composition of particles’ surfaces and also the environment, such as pH, concentration of the ions, and small molecule additives. Typically, in a diluted aqueous system, the concentration of the particles and the zeta potential are not significantly related. However, when the concentration of the particles exceeds a critical point, it is necessary to consider the effect on zeta potential value contributed by the stronger particle-particle interactions and a larger number of charged ions in the solvent environment.
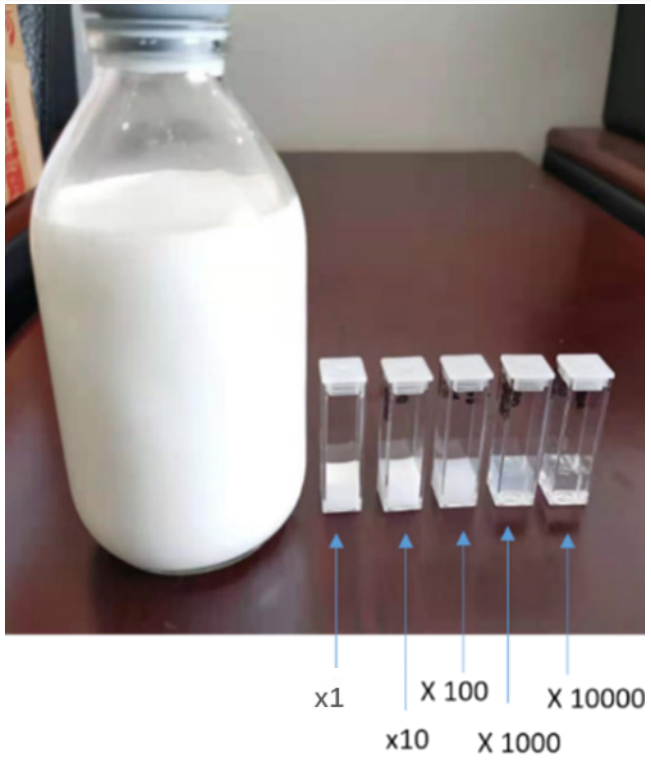
As a result, measuring and interpreting the zeta potential of highly concentrated samples have always been challenges. In this application note, the zeta potentials of fat emulsion dispersed in water at different concentrations are measured by the BeNano 180 Zeta (Bettersize Instrument Ltd.). Made up of soybean oil, glycerides, fatty acids, and phospholipids, fat emulsion is typically opaque with a milky-white color. The folded capillary cell compatible with the BeNano 180 Zeta has a light path as short as 4 mm, which allows for zeta potential measurement even for concentrated samples.
Theory and Instrumentation
The technology utilized to measure zeta potential is called electrophoretic light scattering (ELS). In an ELS experiment, a laser beam irradiates the sample, where the scattered light is detected at a forward angle of 12°. The sample solution or suspension is subjected to an electric field applied to both ends of the sample cell, resulting in the electrophoretic movement of the charged particles in the sample. Consequently, the scattered light experiences a frequency shift compared to the incident light due to the Doppler effect. The scattered light signals with a frequency shift are converted to a phase shift using PALS analysis. By the phase plot, the velocity of electrophoretic movement per unit electric field, which is denoted as the electrophoretic mobility µ, is obtained. Through Henry’s equation, one can relate the electrophoretic mobility µ and its zeta potential ζ as follows:
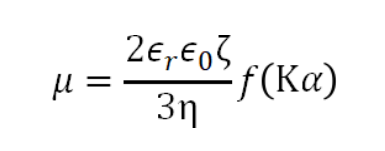
where ε0 is the solvent dielectric constant in vacuum, εr is the relative dielectric constant, η is the solvent viscosity, f(Κα) is the Henry function, Κ is the reciprocal Debye length, α is the particle radius, and Κα refers to the ratio between the thickness of the double layer and the particle radius.
The BeNano 180 Zeta is used for the zeta potential measurement in this application note. A laser beam with a wavelength of 671 nm and a power of 50 mW illuminates the sample. An avalanche photodiode (APD) detector is set up to collect scattered light signals. By using the PALS technique, the BeNano 180 Zeta is efficient at detecting the zeta potential of samples even with low electrophoretic mobility.
Experiment
Since there was no trace of salt (which could dissociate into cations and anions, potentially altering the zeta potential values) found in the ingredients of the fat emulsion, the distilled water was used to dilute the 20% w/v stock fat emulsion down to different concentrations. The diluted fat emulsion suspensions had concentrations ranging from 0.002% to 20% w/v. The temperatures of suspensions were controlled with the built-in temperature control unit to be 25°C. The zeta potential measurements were performed using the folded capillary cell. Each sample was measured at least three times to investigate the repeatability of the results.
Results and Discussion
The zeta potentials of fat emulsion at different concentrations are shown in Figure 2 below.
As can be seen from Figure 1, concentrated fat emulsion suspensions, whose concentrations were from 2% to 20%, had zeta potentials approximately around -5 to -7 mV. For those suspensions whose concentrations were below 2% but above 0.5% m/v, as concentrations decreased, the absolute values of zeta potentials increased. For suspensions with concentrations between 0.002% to 0.5% w/v, the zeta potentials were around -41 to -44 mV. Such small fluctuations indicate that the zeta potentials were independent of concentration in this concentration range.
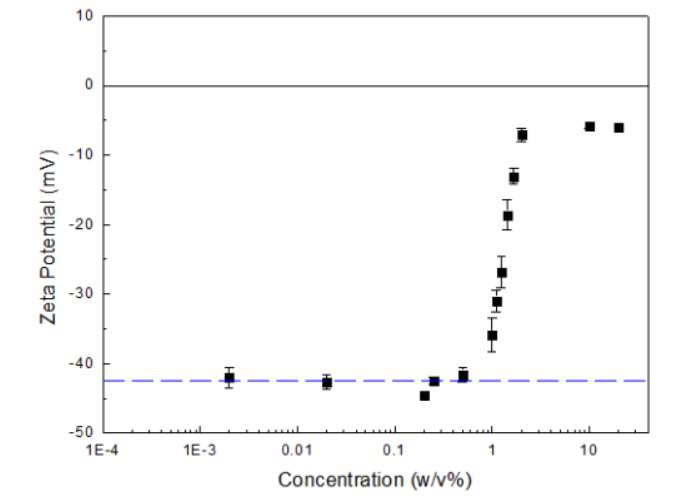
There are two possible reasons that account for the extremely low zeta potentials in highly concentrated fat emulsion suspensions. The first one pertains to the particle-particle interaction. In concentrated suspensions, the particle-particle interaction is so strong that it suppresses the electrophoretic movements, and therefore results in a decrease in electrophoretic mobility of the particles, and thus a decrease in the zeta potential. The second reason pertains to the surface charges of lipid particles. When particle concentrations are high enough, the contribution of charged particles to the ionic strength of the solvent environment can no longer be neglected, leading to a decrease in the zeta potentials. On the other hand, as the suspensions became less concentrated, the two above-mentioned phenomena were less dominant, thus yielding the more accurate and stable zeta potential results.
Actually, zeta potentials of suspensions with concentrations higher than 0.5% were no longer the true zeta potential of the system but were instead the apparent zeta potential, which cannot reflect the actual potential value of the suspension system. To obtain the true zeta potential value of an aqueous system, it is essential to use a proper dilutant, which keeps the environment as same as the stock solution, to dilute the stock solution to an appropriate concentration range where the zeta potential does not depend on the concentration.
Conclusions
The zeta potentials of fat emulsion suspensions at different concentrations were successfully characterized by the ELS technology of the BeNano 180 Zeta. The results confirm the capability of the BeNano 180 Zeta in measuring the zeta potential of highly concentrated samples thanks to the innovative optical system and the folded capillary cell with a short light path. It is also concluded that the zeta potential results obtained from highly concentrated samples could not reflect the true potential value of the system. In order to obtain the true zeta potential results, use a proper dilutant to dilute the concentrated sample to an appropriate range. For an unknown aqueous system, it is recommended to perform a concentration titration experiment to determine the optimal concentration range.
About the Authors
 |
Zhibin Guo Application Manager @ Bettersize Instruments |
 |
Dr. Ning Chief Product Officer @ Bettersize Instruments |
|
Advanced Nanoparticle Size & Zeta Potential Analysis
|
|
Recommended articles
Rate this article
