The significance of particle size analysis for API
2020-09-17Application Briefs
Drug Quality and Control
Drug quality depend on lots of parameters. The dissolution rate of the drug into the human body is a major consideration of the drug quality and control thereof. Dissolution rate refers to the rate of the drug absorption into the tissue and organ. In solid drug preparation, the drug absorbed by the human body must go through the process of disintegration and dissolution. If the drug does not easily release into the body or the dissolution rate of the drug is extremely slow, there will be a problem accepting that particular drug. On the other hand some drugs have an overly rapid pharmacological action which can render them unsafe. If the drug dissolves too fast, it can drastically reduce the time that it can be effective in the human body, but not only that it may also produce significant adverse reactions as well. In this case, the preparation of drug dissolution rate control is particularly important. The dissolution degree of the drug in the solvent are expressed by the dissolution rate. A tablet is a common dosage form for oral reception, and the dissolution rate is closely related to the particle size distribution of the raw and excipient materials, prescription composition, particle hardness and processing conditions. In the production of active pharmaceutical ingredient (API), particle size is an indispensable parameter. Depending on the requirements of different tablet’s dissolution, the particle size of the API should be controlled in different particle size segments to achieve maximum efficacy.
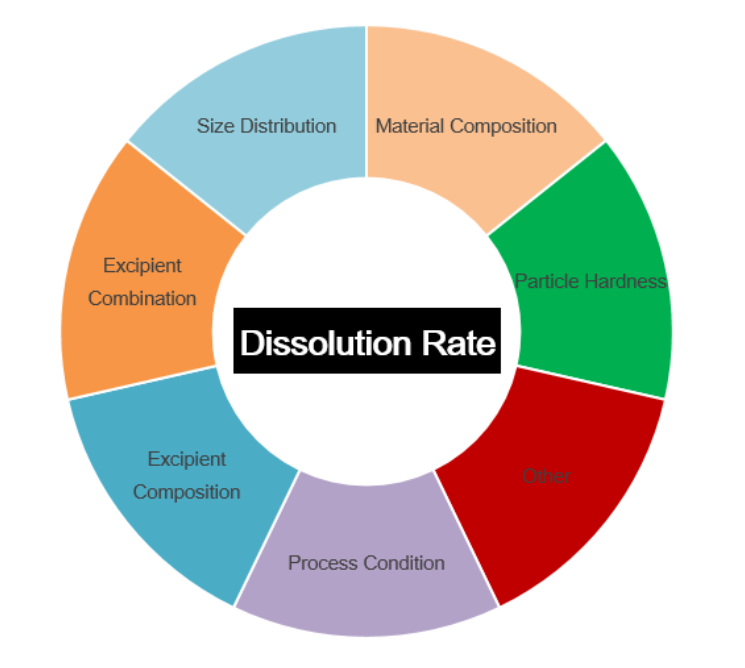
Figure 1. Relationship between properties of drug properties and dissolution rate
Dissolution Rate
Dissolution rate is a key evaluation parameter for drug R&D facilities to optimize the tablet formulation process and also for regulatory authorities to monitor the tablet quality and efficacy. It is the primary prediction method for drug bioavailability and bioequivalence. In the view of China's International Coordination Committee and amongst many other countries throughout the world regarding the most important technical Requirements for human drug registration, the preparation process has become the focus of attention and the focus of demand for pharmaceutical research institutions. No matter whether it concerns the development of generic or patented drugs, they cannot deviate from the consistency evaluation, the research on drug dissolution is indispensable now and in our lifetime for the future.
Apart from tablets, inhalation preparation of API particle size will directly affect the dose uniformity and deposition efficiency in the lungs. Formulations that have a particle size which is too large in size will impact on the throat and not arrive at the lungs, whereas those that are too small will enter the lungs and not stay there, being exhaled into the atmosphere with the optimum size being between 1 to 7 microns. In intravenous preparations, large insoluble particles will directly affect the safety of the formulation. The Pharmaceutical excipient, particle size, morphology and liquidity will directly affect the formability, uniformity and disintegration, dissolution, tablet hardness and appearance of preparation. Therefore, worldwide pharmacopoeia see drug particle detection and evaluation as a very important property parameter.
Laser Diffraction Particle Size Distribution Analysis in Famotidine
Famotidine is used in many dosage forms, including tablets, capsules and injectables. Famotidine is a Histamine H2 receptor antagonist, which can inhibit the secretion of gastric acid. It is suitable for gastric and duodenal ulcers, reflux esophagitis, upper gastrointestinal hemorrhage, Zollinger-Ellison syndrome and other diseases. The laser diffraction particle size distribution measurement of raw and excipients for this product has been recorded in the pharmacopoeias of many countries. The particle size distribution is calculated by the laser diffraction technique, whereby the Mie theoretical model calculates the particle size distribution from the light intensity each detector receives after it is de-convoluted. Here we can see the particle size distribution gleaned from a laser diffraction analysis of Famotidine.
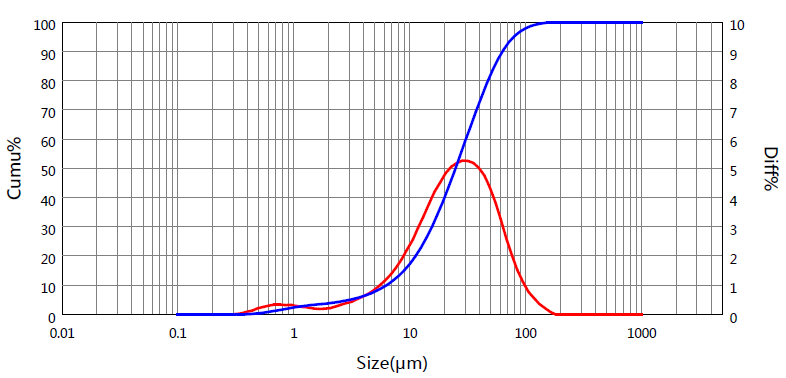
Figure 2. Particle size distribution diagram of single Famotidine sample
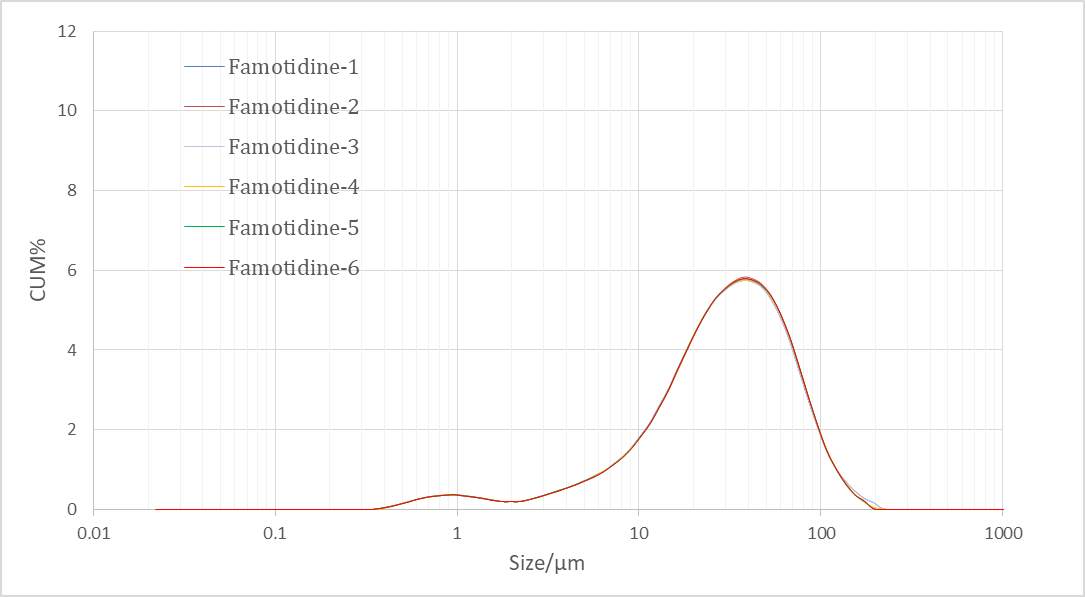
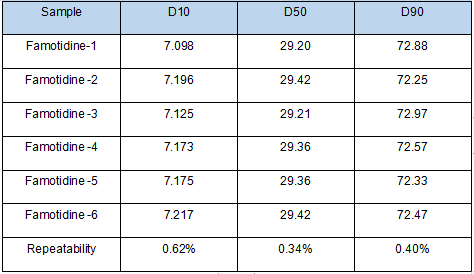
Figure 3. Particle size analysis of Famotidine samples by Bettersizer 2600 measurement
Image Analysis in Famotidine


Figure 5. Image analysis of Famotidine-2 in BeVision S1 measurement
Under image analysis the morphology and the structure of the particles can be directly observed and created by mathematical models and complex algorithms. Bettersize have made the measurement technology simple and intuitive to enable every user to become an expert.
From the observation of the microscope, the dispersion effect of the two samples is good, and there is no agglomeration phenomenon. The particles are almost all needle-like translucent particles, and the large particles are between 100 and 200um, which is consistent with the data measured by the laser particle size analyzer.
Conclusion
Laser diffraction is a powerful technology widely used in particle size detection of raw and excipient materials. Meanwhile, this method also has the advantages of fast detection speed and high precision. However, laser diffraction technology has insurmountable disadvantages. Laser diffraction technology can not directly determine the size and shape of active pharmaceutical ingredient in a whole capsule and tablet. The laser diffraction can only show the size distribution of whole ingredient of drug as above Famotidine. Therefore, use the image analysis to give a cross validation is very necessary. Under the image analysis system, the size of final particles can be intuitively confirmed, and whether the sample is agglomerated or broken cannot be known. And for many preparations, the shape of the primary and excipient crystals is also critical. With the combination of this two technique, it can not only receive the size distribution for the dissolution rate research base on the laser diffraction, but also can have a great direction in optimize the drug formula process base on the image analysis. Drug quality is not easy to control, but Bettersize always can give a great solution in particle for pharmaceutical research and development.



