In the built-in temperature control system of the Nanoptic 90 Plus, the default test temperature is controlled to be 25℃. For these three samples, we set the temperature at 37℃ to simulate the temperature environment of human bodies.
Each sample was measured at least three times after it was placed in the sample cell, in order to check the repeatability.
Results and Discussion
By analyzing the scattered signals of the samples, we obtained the correlation functions of these samples:
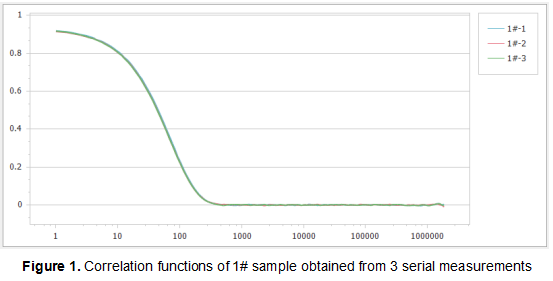
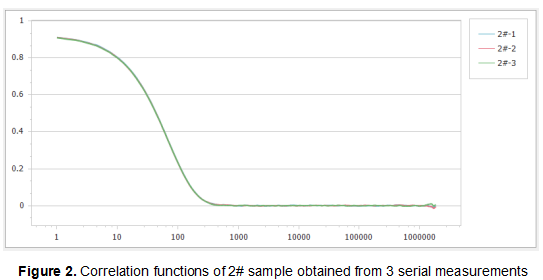
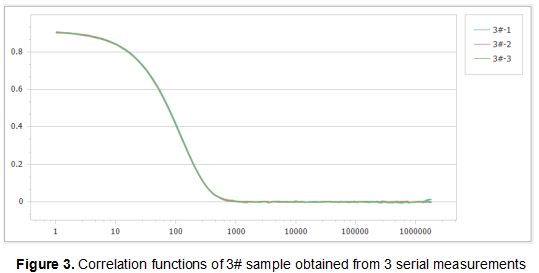
Figure 1, Figure 2 and Figure 3 show the correlation functions of the three samples. It can be seen that the repeatability of the results is excellent, which illustrates the high sensitivity and stability of the Nanoptic 90 Plus optical system.
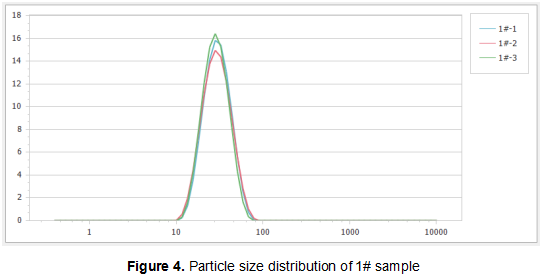
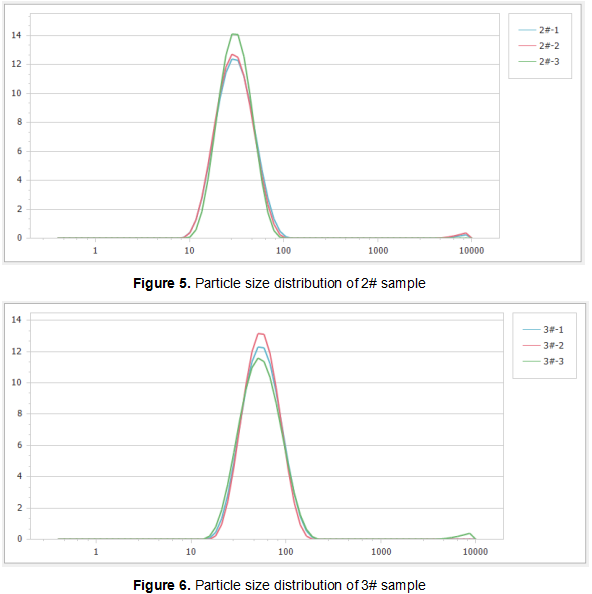
Figure 4, Figure 5 and Figure 6 show the particle size distributions of the three samples in multiple measurements. It can be seen that the particle size distribution of each sample under such measurement conditions has reached good repeatability. Among them, a very small amount of coarse cyclosporin A aggregates were found in the distribution curves of sample 2# and sample 3#. The small content of these components may not affect the oral bioavailability of the drug, but the presence of a small amount of aggregates probably indicates the instability of the system. Therefore, it is worthwhile to continue characterizing the samples in future research, by observing whether the size and size distribution of these agglomerates change over time.
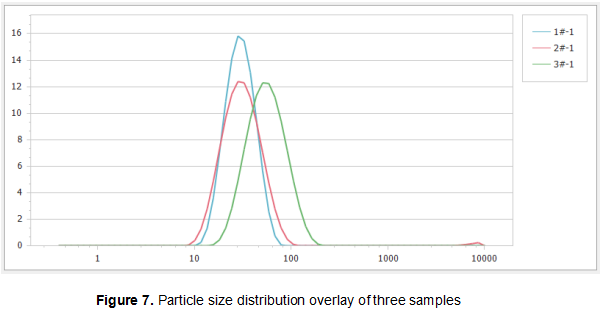
In Figure 7, the particle size distributions of the three samples are overlapped, and it can be seen that the particle size distribution of the 3# sample is significantly higher than the 1# sample and the 2# sample.
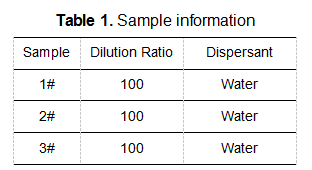
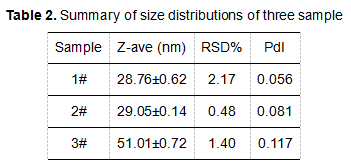
The results of multiple tests of samples are shown in the table below:
From the particle size results, we can see the difference in the Z-average size of the three samples. The particle size of the 1# sample and the 2# sample are similar, whereas the particle size of the 3# sample is significantly larger than the first two samples. The relative standard deviation of repeated measurements is less than 3%, which shows that all samples are stable during the measurement. Through the distribution coefficient (PdI), we can see that for 3# sample, not only the size result is biggest, but also the PdI value significantly exceeds other samples, which may be related to the particular manufacturing process of the formulation.
Reference
[1] By Ben Mills - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=3644616
[2] By Yikrazuul - Own work, Public Domain, https://commons.wikimedia.org/w/index.php?curid=4318711
[3] Klyashchitsky, B.A., Owen, A.J., 1998. Drug delivery systems for cyclosporine: achievements and complications. J. Drug Target 5, 443–458.